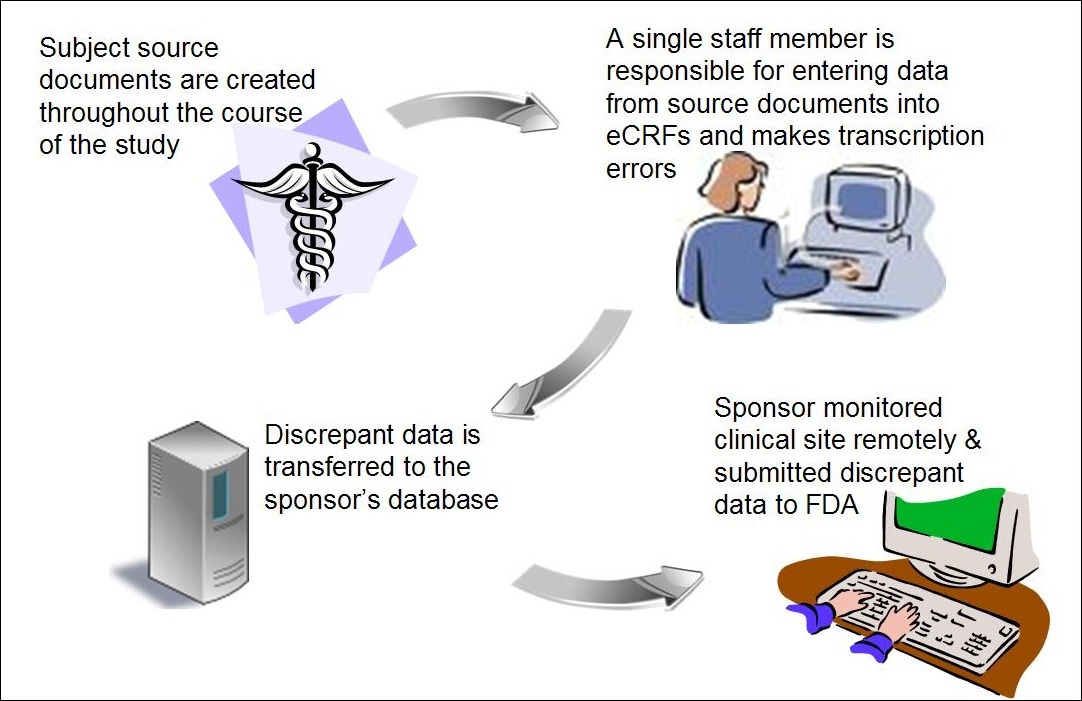
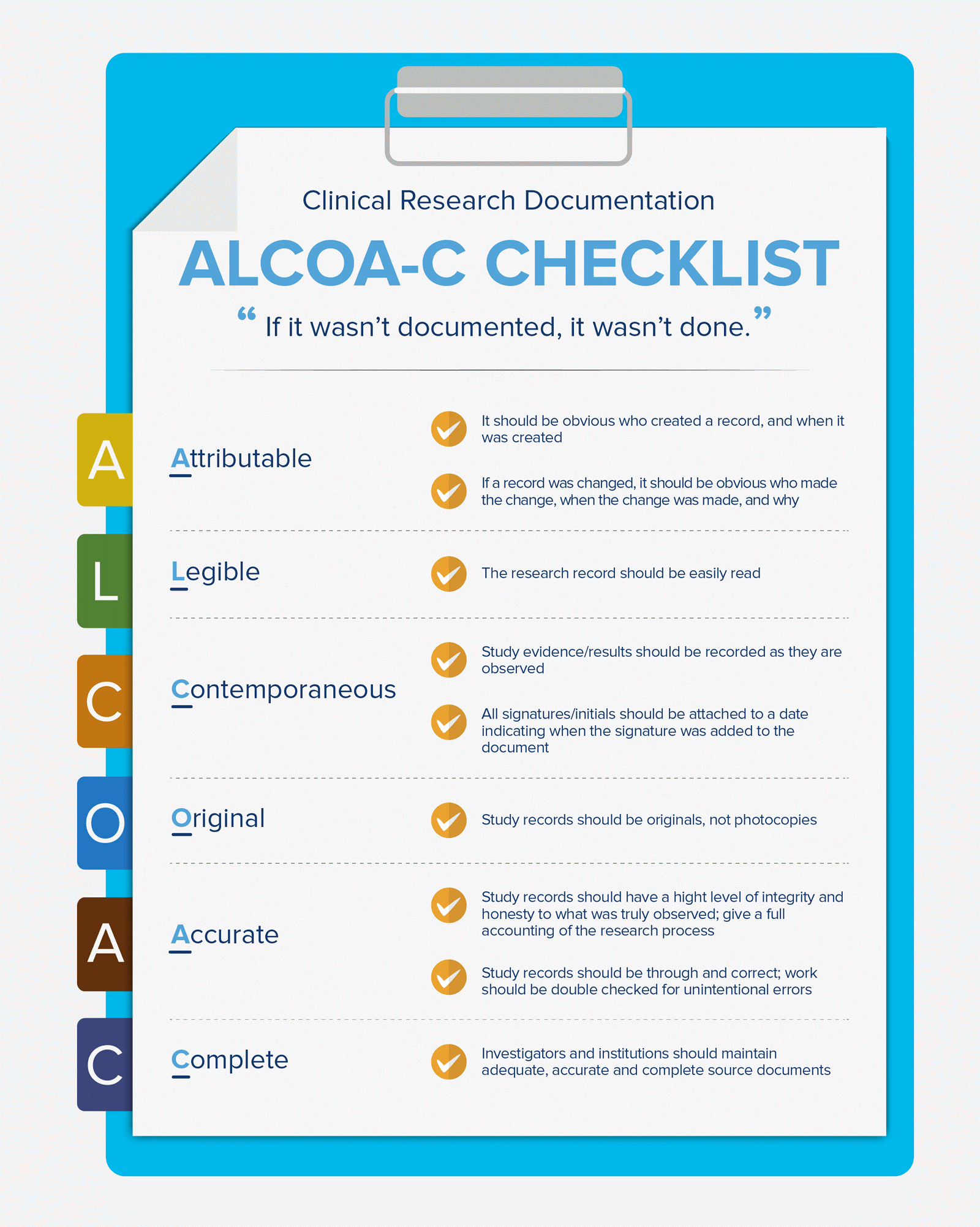
Product code: What is source document in 2025 clinical trials
Clinical Trial Source Doc Definition PPT 2025, Clinical Trial Source Doc Definition PPT 2025, Source Documentation 101 Research In Action Advancing Health 2025, Importance and Purpose of Source Documents in Clinical Studies 2025, Study Documentation Example of a Source Document EUPATI Open 2025, Untitled Document 2025, what is a source document Clinical Research Certification I Blog 2025, Clinical Trial Source Doc Definition PPT 2025, Documentation clinical trial PPT 2025, PDF Source Documents for Clinical Trial Visits 2025, PPT Source Documentation PowerPoint Presentation free download 2025, Untitled Document 2025, what is a source document Clinical Research Certification I Blog 2025, Source Documents Slide Share PPT 2025, Research Documentation Requirements Best Practices 2025, The Future of Clinical Trials Using Electronic Data Capture 2025, ALCOA C Conduct Science 2025, Research Professionals Network Workshop Series 2025, The Power of Source Documents 2025, PPT CLINICAL TRIALS 101 PowerPoint Presentation free download 2025, Clinical Trial Source Doc Definition PPT 2025, Source Documentation. Objectives At the conclusion of this 2025, Clinical Research Translation Services Language Scientific 2025, Source Documents and CRFs 2025, Study Start Up and Implementation ppt download 2025, PPT Orientation for New Clinical Research PERSONNEL Module 2 2025, Why Documentation Is Important In Clinical Research Fineness 2025, Clinical Trial Source Doc Definition PPT 2025, Data Management eSource Services EPS Corporation 2025, Research Professionals Network Workshop Series 2025, Data collection query and source data verification process using 2025, Relejuvant Clinical Services Definition of Source Document in 2025, Data Management in Clinical Trials ScienceDirect 2025, Review of source documents Review of source documents Background 2025, Clinical Trial Source Doc Definition PPT 2025, Source documentation in clinical trials Clinical Research Info 2025, Quality Control in Clinical Trials BioPharma Services 2025, The Power of Source Documents 2025, Essential Documents Source Documentation SOPs ppt download 2025, create source document for clinical trial 2025.
Clinical Trial Source Doc Definition PPT 2025, Clinical Trial Source Doc Definition PPT 2025, Source Documentation 101 Research In Action Advancing Health 2025, Importance and Purpose of Source Documents in Clinical Studies 2025, Study Documentation Example of a Source Document EUPATI Open 2025, Untitled Document 2025, what is a source document Clinical Research Certification I Blog 2025, Clinical Trial Source Doc Definition PPT 2025, Documentation clinical trial PPT 2025, PDF Source Documents for Clinical Trial Visits 2025, PPT Source Documentation PowerPoint Presentation free download 2025, Untitled Document 2025, what is a source document Clinical Research Certification I Blog 2025, Source Documents Slide Share PPT 2025, Research Documentation Requirements Best Practices 2025, The Future of Clinical Trials Using Electronic Data Capture 2025, ALCOA C Conduct Science 2025, Research Professionals Network Workshop Series 2025, The Power of Source Documents 2025, PPT CLINICAL TRIALS 101 PowerPoint Presentation free download 2025, Clinical Trial Source Doc Definition PPT 2025, Source Documentation. Objectives At the conclusion of this 2025, Clinical Research Translation Services Language Scientific 2025, Source Documents and CRFs 2025, Study Start Up and Implementation ppt download 2025, PPT Orientation for New Clinical Research PERSONNEL Module 2 2025, Why Documentation Is Important In Clinical Research Fineness 2025, Clinical Trial Source Doc Definition PPT 2025, Data Management eSource Services EPS Corporation 2025, Research Professionals Network Workshop Series 2025, Data collection query and source data verification process using 2025, Relejuvant Clinical Services Definition of Source Document in 2025, Data Management in Clinical Trials ScienceDirect 2025, Review of source documents Review of source documents Background 2025, Clinical Trial Source Doc Definition PPT 2025, Source documentation in clinical trials Clinical Research Info 2025, Quality Control in Clinical Trials BioPharma Services 2025, The Power of Source Documents 2025, Essential Documents Source Documentation SOPs ppt download 2025, create source document for clinical trial 2025.