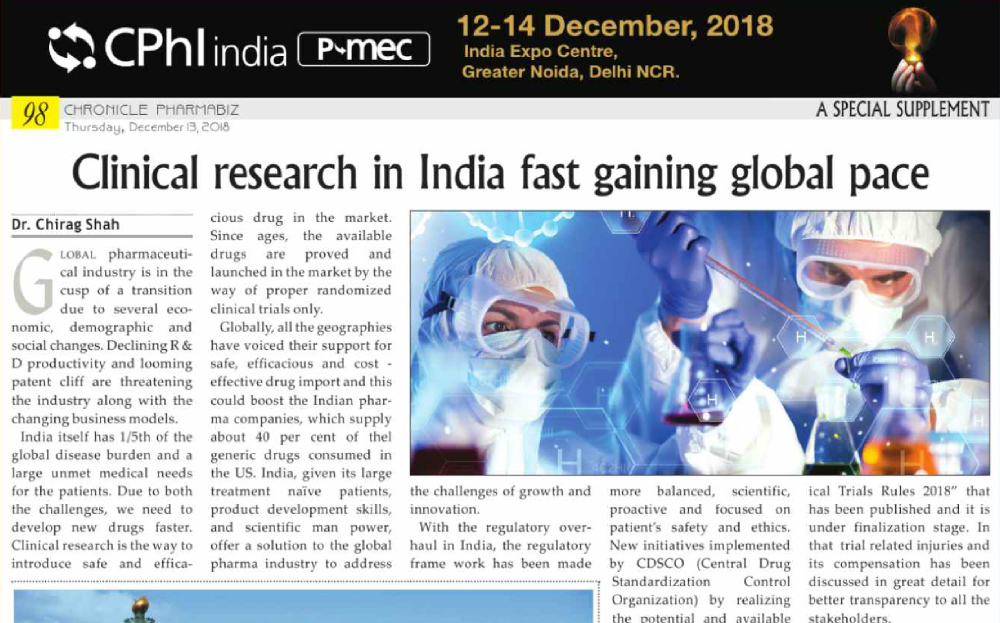
Product code: Ind is initiatied to support 2025 clinical development
Clinical trials in India Wikipedia 2025, Current clinical trial approval process in India. Abbreviations 2025, Year Ender 2020 Department of Biotechnology DBT M o S T 2025, India s rise as a clinical trials destination and the 2025, After a lull of five years clinical trials on the rise in India 2025, Prasar Bharati News Services The ICMRDELHI has partnered with 2025, Public Health Foundation of India PHFI 2025, Make In India Prime Minister of India 2025, ICMR on X 2025, Global Gene Therapy Initiative Impact Project Caring Cross 2025, An overview of clinical decision support systems benefits risks and 2025, Balaji Veeraraghavan Kamini Walia write We ve successfully made 2025, Home Principal Scientific Adviser 2025, Department of Biotechnology India Department of 2025, DEFA14A 2025, Clinical trial Wikipedia 2025, News Events Cliantha Research A full service Clinical 2025, Make In India Prime Minister of India 2025, ARMED FORCES MEDICAL COLLEGE Department of Medical Research The 2025, Common Problems to Avoid with IND Applications for New Drugs and 2025, UPSC Preparation Current Affairs Government Schemes Make in India 2025, Student Fellowships Skill Development Department of Biotechnology 2025, PDF Pediatric Neuropalliative Care in India Current Landscape 2025, Diversity Inclusion in Clinical Trials 2025, Wolters Kluwer Empowering Indian Healthcare with Clinical 2025, Drishti IAS PDF 2025, C DAC Centre for Development of Advanced Computing India 2025, AI Is Big Pharma s Latest Tool to Boost Equality in Clinical 2025, The present and future of bispecific antibodies for cancer therapy 2025, Serum Institute of India to make 240 mn investments in UK Mint 2025, Evolving Clinical Development Regulatory Framework in India Veeda 2025, Press Information Bureau 2025, Clinical Trials Regulation European Medicines Agency 2025, Regulation in clinical trial Schedule Y and recent amendments PPT 2025, Dr. Swati Sinha on LinkedIn pharma covid19 vaccine 2025, India eyes global front runners in Covid 19 vaccine plan Latest 2025, Clinical trial participation should be offered as a care option by 2025, Indian Society for Clinical Research ISCR 17th annual conference 2025, Indian Economy Growth Rate Economic System Insights IBEF 2025, Evolving Clinical Development Regulatory Framework in India Veeda 2025.
Clinical trials in India Wikipedia 2025, Current clinical trial approval process in India. Abbreviations 2025, Year Ender 2020 Department of Biotechnology DBT M o S T 2025, India s rise as a clinical trials destination and the 2025, After a lull of five years clinical trials on the rise in India 2025, Prasar Bharati News Services The ICMRDELHI has partnered with 2025, Public Health Foundation of India PHFI 2025, Make In India Prime Minister of India 2025, ICMR on X 2025, Global Gene Therapy Initiative Impact Project Caring Cross 2025, An overview of clinical decision support systems benefits risks and 2025, Balaji Veeraraghavan Kamini Walia write We ve successfully made 2025, Home Principal Scientific Adviser 2025, Department of Biotechnology India Department of 2025, DEFA14A 2025, Clinical trial Wikipedia 2025, News Events Cliantha Research A full service Clinical 2025, Make In India Prime Minister of India 2025, ARMED FORCES MEDICAL COLLEGE Department of Medical Research The 2025, Common Problems to Avoid with IND Applications for New Drugs and 2025, UPSC Preparation Current Affairs Government Schemes Make in India 2025, Student Fellowships Skill Development Department of Biotechnology 2025, PDF Pediatric Neuropalliative Care in India Current Landscape 2025, Diversity Inclusion in Clinical Trials 2025, Wolters Kluwer Empowering Indian Healthcare with Clinical 2025, Drishti IAS PDF 2025, C DAC Centre for Development of Advanced Computing India 2025, AI Is Big Pharma s Latest Tool to Boost Equality in Clinical 2025, The present and future of bispecific antibodies for cancer therapy 2025, Serum Institute of India to make 240 mn investments in UK Mint 2025, Evolving Clinical Development Regulatory Framework in India Veeda 2025, Press Information Bureau 2025, Clinical Trials Regulation European Medicines Agency 2025, Regulation in clinical trial Schedule Y and recent amendments PPT 2025, Dr. Swati Sinha on LinkedIn pharma covid19 vaccine 2025, India eyes global front runners in Covid 19 vaccine plan Latest 2025, Clinical trial participation should be offered as a care option by 2025, Indian Society for Clinical Research ISCR 17th annual conference 2025, Indian Economy Growth Rate Economic System Insights IBEF 2025, Evolving Clinical Development Regulatory Framework in India Veeda 2025.


.jpg/640px-Clinical_Trial_Participant_Receives_Injection_(34033294061).jpg)