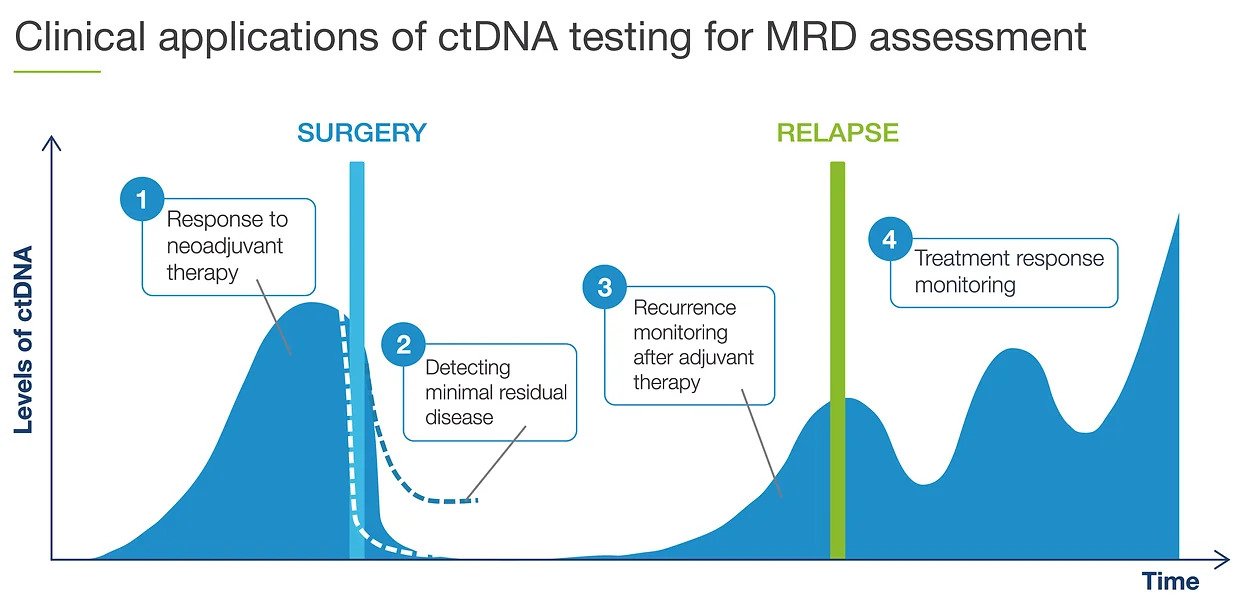
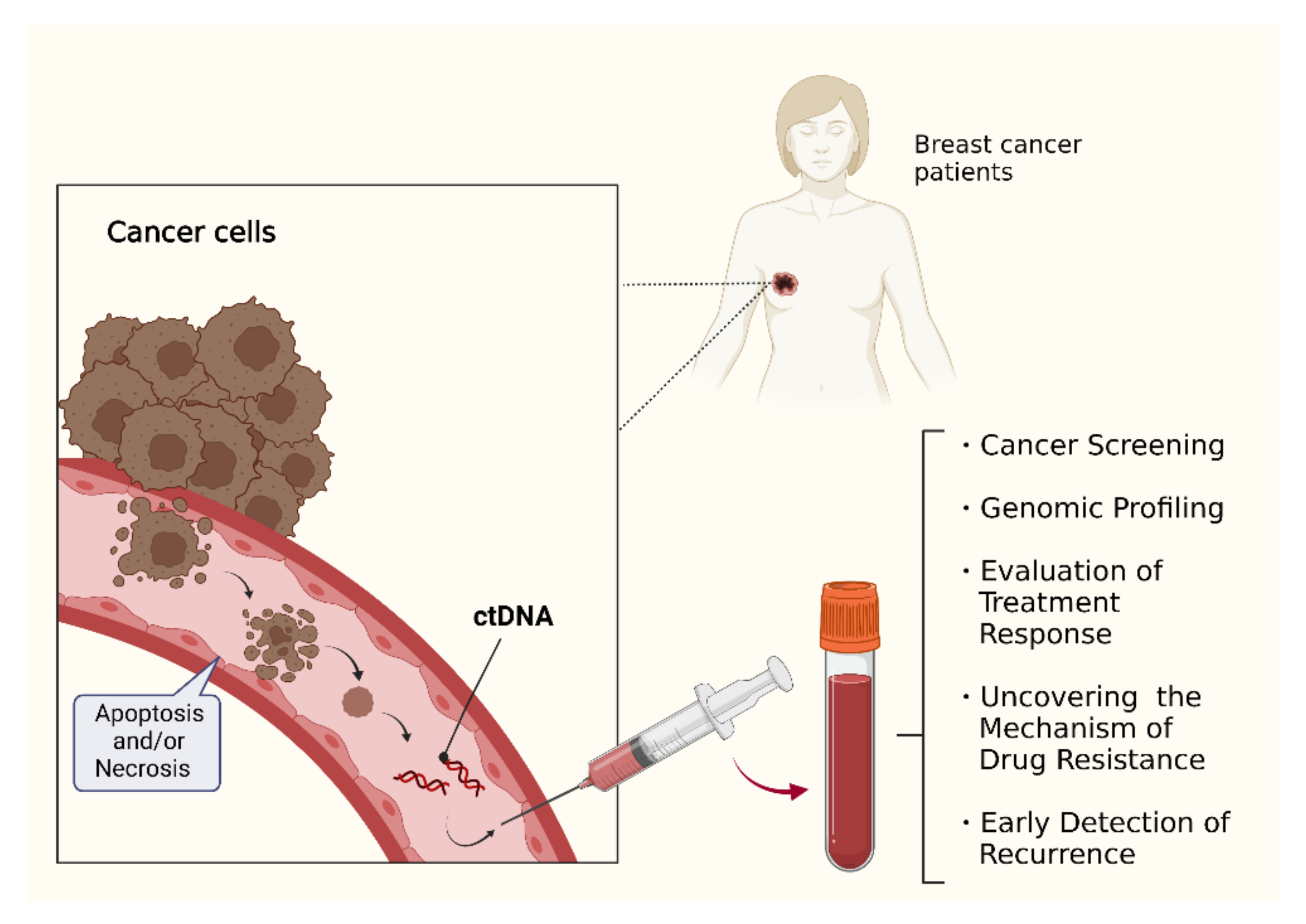
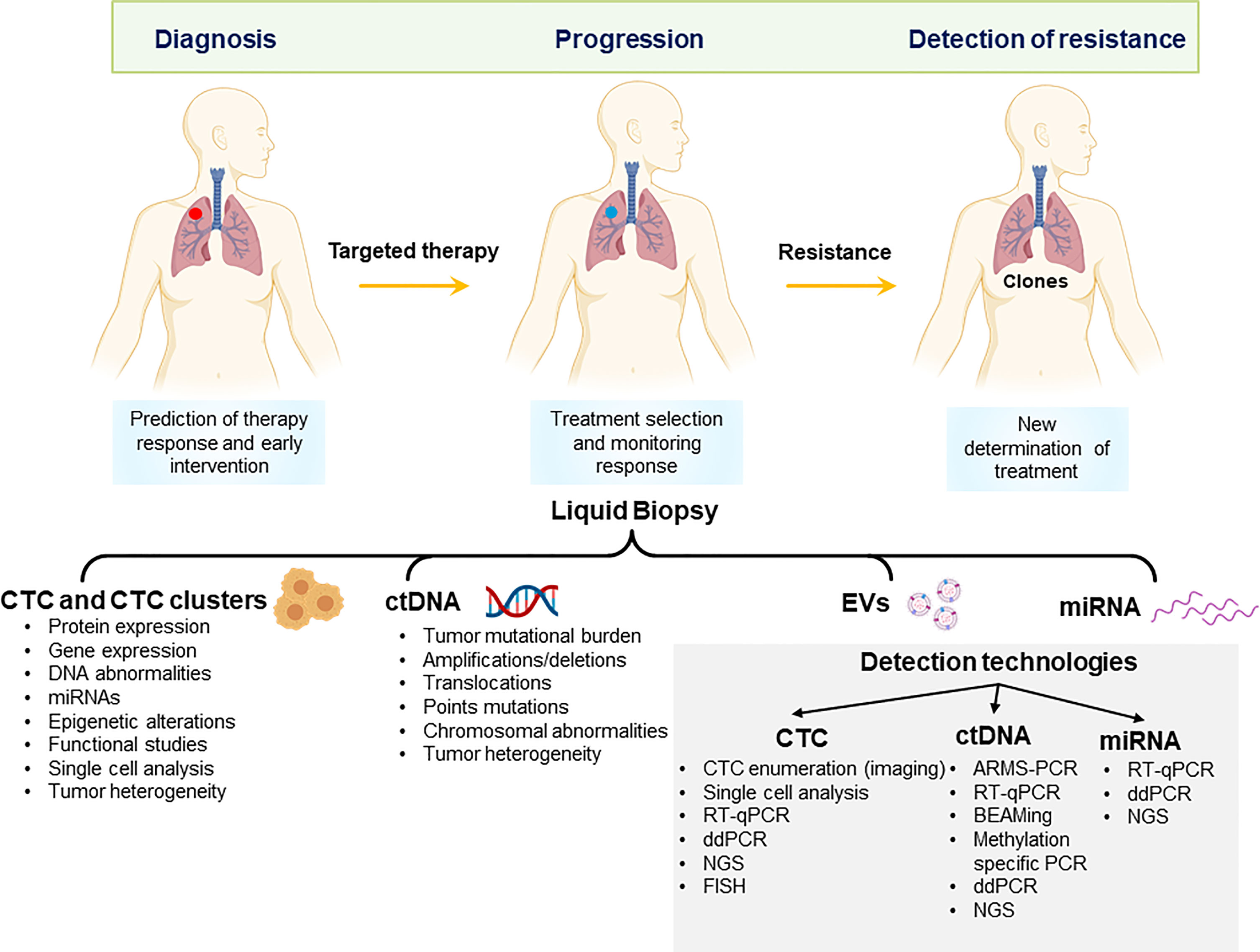
Product code: Is ctdna 2025 testing clinical approved
Examples of FDA approved commercial tests for clinical application 2025, Examples of FDA approved commercial tests for clinical application 2025, IJMS Free Full Text Using cfDNA and ctDNA as Oncologic Markers 2025, Integrating circulating free DNA cfDNA analysis into clinical 2025, Cancers Free Full Text Implementing ctDNA Analysis in the 2025, Clinical relevance of blood based ctDNA analysis mutation 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Frontiers Clinical applications of circulating tumor derived DNA 2025, Circulating tumor DNA validity and potential uses in metastatic 2025, IJMS Free Full Text Circulating Tumor DNA in Precision 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Signatera Unilabs 2025, Processes Free Full Text Circulating Tumor DNA in Oncology 2025, Frontiers Clinical Applications of Circulating Tumour Cells and 2025, Clinical significance and biology of circulating tumor DNA in high 2025, The Utility of Liquid Biopsy in Oncology Clinical Trials Xtalks 2025, Clinical correlates of ctDNA. A Biomarker discovery rate for 2025, Circulating tumor DNA and liquid biopsy in oncology Nature Cancer 2025, Circulating Tumor DNA Test Clinically Validated molecular 2025, Circulating tumor DNA in cancer diagnosis monitoring and prognosis 2025, Glass half full Improving outcomes in patients with circulating 2025, Predicting immunotherapy responses using circulating tumor DNA 2025, Cancer Research and Treatment 2025, What is circulating tumor DNA and how is it used to diagnose and 2025, JMP Free Full Text Liquid Biopsy in Advanced Colorectal Cancer 2025, Prevalence of pathogenic germline variants in the circulating 2025, Circulating Tumor DNA an overview ScienceDirect Topics 2025, DNA Shed From Colon Cancers Into Bloodstream Guide Chemotherapy 2025, The application of ctDNA in clinical trials across different 2025, Cancer Research and Treatment 2025, MOA 2025, Variant allele frequency in circulating tumor DNA correlated with 2025, FDA drafts guidance on ctDNA Streck 2025, Frontiers Clinical Applications of Liquid Biopsy in Gastric Cancer 2025, Cell free DNA analysis in current cancer clinical trials a review 2025, Liquid Biopsy Test Protocol and Steps Technology Networks 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Circulating cell free DNA for cancer early detection ScienceDirect 2025, ctdna Archives Genes2Me Blog CE IVD Approved RT PCR POC 2025, Recurrence monitoring using ctDNA in patients with metastatic 2025.
Examples of FDA approved commercial tests for clinical application 2025, Examples of FDA approved commercial tests for clinical application 2025, IJMS Free Full Text Using cfDNA and ctDNA as Oncologic Markers 2025, Integrating circulating free DNA cfDNA analysis into clinical 2025, Cancers Free Full Text Implementing ctDNA Analysis in the 2025, Clinical relevance of blood based ctDNA analysis mutation 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Frontiers Clinical applications of circulating tumor derived DNA 2025, Circulating tumor DNA validity and potential uses in metastatic 2025, IJMS Free Full Text Circulating Tumor DNA in Precision 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Signatera Unilabs 2025, Processes Free Full Text Circulating Tumor DNA in Oncology 2025, Frontiers Clinical Applications of Circulating Tumour Cells and 2025, Clinical significance and biology of circulating tumor DNA in high 2025, The Utility of Liquid Biopsy in Oncology Clinical Trials Xtalks 2025, Clinical correlates of ctDNA. A Biomarker discovery rate for 2025, Circulating tumor DNA and liquid biopsy in oncology Nature Cancer 2025, Circulating Tumor DNA Test Clinically Validated molecular 2025, Circulating tumor DNA in cancer diagnosis monitoring and prognosis 2025, Glass half full Improving outcomes in patients with circulating 2025, Predicting immunotherapy responses using circulating tumor DNA 2025, Cancer Research and Treatment 2025, What is circulating tumor DNA and how is it used to diagnose and 2025, JMP Free Full Text Liquid Biopsy in Advanced Colorectal Cancer 2025, Prevalence of pathogenic germline variants in the circulating 2025, Circulating Tumor DNA an overview ScienceDirect Topics 2025, DNA Shed From Colon Cancers Into Bloodstream Guide Chemotherapy 2025, The application of ctDNA in clinical trials across different 2025, Cancer Research and Treatment 2025, MOA 2025, Variant allele frequency in circulating tumor DNA correlated with 2025, FDA drafts guidance on ctDNA Streck 2025, Frontiers Clinical Applications of Liquid Biopsy in Gastric Cancer 2025, Cell free DNA analysis in current cancer clinical trials a review 2025, Liquid Biopsy Test Protocol and Steps Technology Networks 2025, A clinician s handbook for using ctDNA throughout the patient 2025, Circulating cell free DNA for cancer early detection ScienceDirect 2025, ctdna Archives Genes2Me Blog CE IVD Approved RT PCR POC 2025, Recurrence monitoring using ctDNA in patients with metastatic 2025.