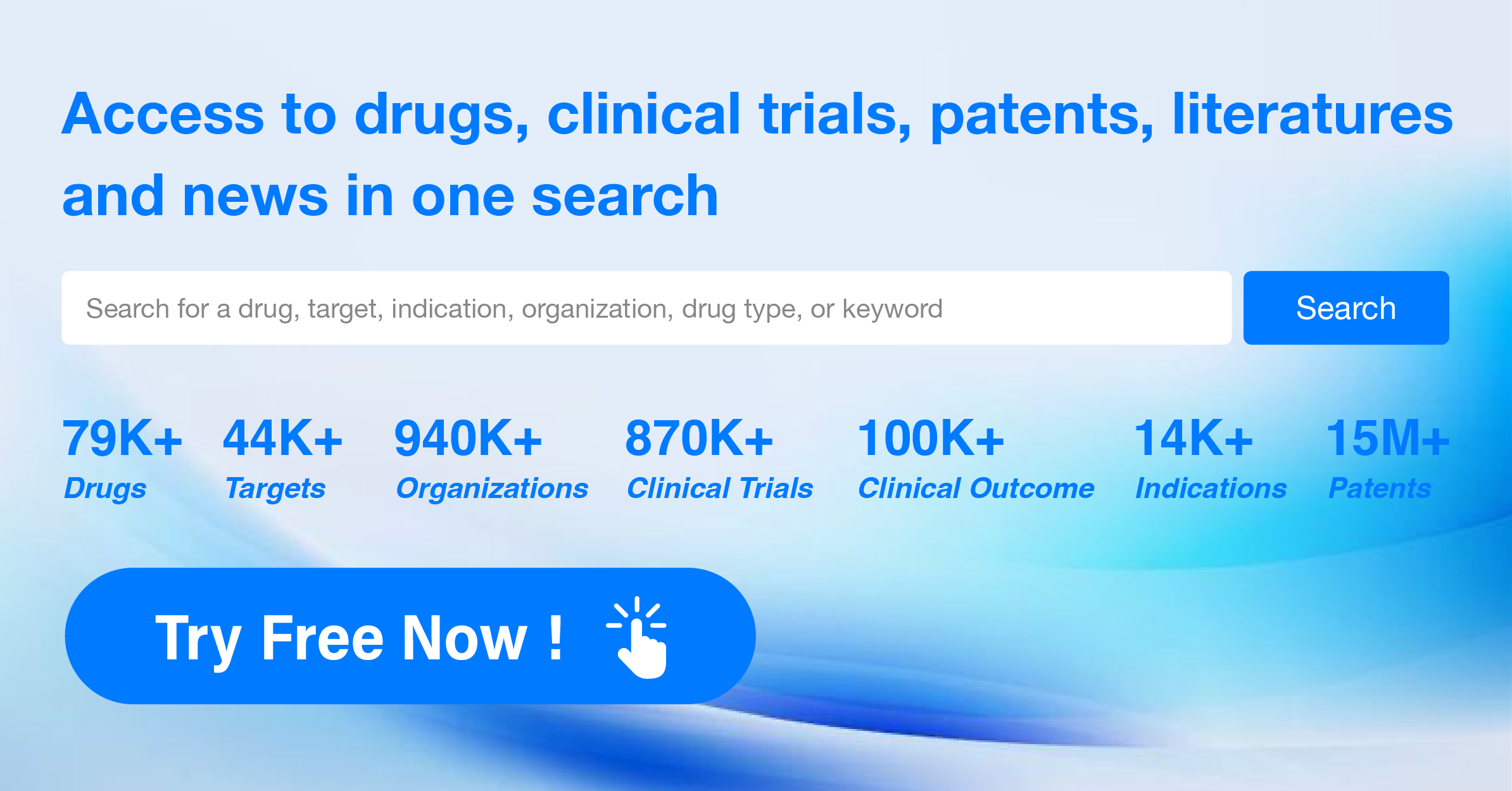
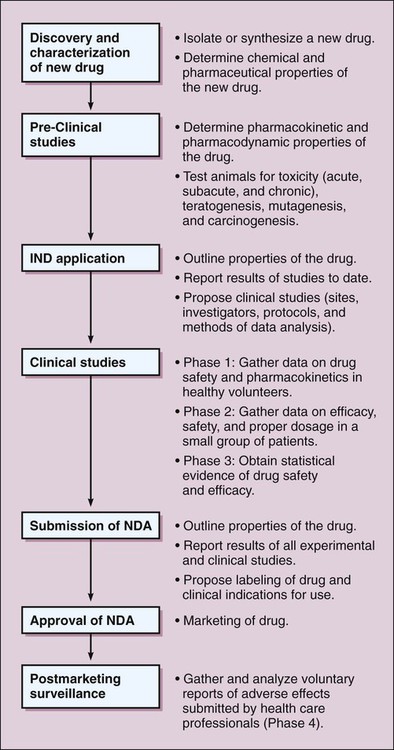
Product code: What is a 2025 drug's clinical indications
Drug Indications Noble Standard 2025, Indications for therapeutic drug monitoring PPT 2025, Frontiers DICE A Drug Indication Classification and 2025, Inferring new drug indications using the complementarity between 2025, A Overview of the selected 87 drugs and their clinical 2025, Clinical indications for the most used drugs in the treatment of 2025, Indication Prioritization Insights Clarivate 2025, Rationale current clinical indications and dosages of COVID 19 2025, Early phase clinical trial played a critical role in the Food and 2025, A standard database for drug repositioning Scientific Data 2025, Drug development Wikipedia 2025, Therapeutic Area vs. Indication Vial 2025, Drugs clinical trials of thyroid diseases in China 09 22 DDDT 2025, Drug misuse and dependence UK guidelines on clinical management 2025, What is a sham in clinical trials 2025, Classification Of Drugs and Clinical Therapeutic 2025, Drug Development and Safety Basicmedical Key 2025, PPT Teicoplanin and vancomycin clinical indications and 2025, Approved Drugs Archives Guideline Central 2025, Clinical Pharmacology powered by ClinicalKey Elsevier 2025, COVID Free Full Text A Comprehensive Review of Drug 2025, What is an IND What is a Clinical Hold Why Do Clinical Holds Happen 2025, Clinical trials in India Wikipedia 2025, US Drug Labels modules for clinical decision support 2025, Frontiers Trends in innovative pediatric drug development in 2025, Indication expansion Blockbuster Drugs Revolutionizing Treatment 2025, Prioritizing Indications An Analytical Framework Equinox Group 2025, PDF The New Antiepileptic Drugs Their Neuropharmacology and 2025, National Drug Authority Guidelines for the Conduct of Clinical Trials 2025, The Ethics of Off Label Drug Use Dimensions of Dental Hygiene 2025, Drug Development Boston Biotech Clinical Research 2025, Unintended Consequences of the Inflation Reduction Act Clinical 2025, Part1 chapter12 2025, PDR Guide to Drug Interactions Side Effects and Indications 2008 2025, Our Drug Pipeline Indication Strategy NW PharmaTech 2025, The Success Rate of Clinical Trials Is Higher than We Thought 2025, Special FDA designations for drug development orphan fast track 2025, Guideline for the management of pediatric off label use of drugs 2025, ReGARDD Regulatory Guidance for Academic Research of Drugs and 2025, Individual Approach to Laboratory Monitoring of Antiepileptic 2025.
Drug Indications Noble Standard 2025, Indications for therapeutic drug monitoring PPT 2025, Frontiers DICE A Drug Indication Classification and 2025, Inferring new drug indications using the complementarity between 2025, A Overview of the selected 87 drugs and their clinical 2025, Clinical indications for the most used drugs in the treatment of 2025, Indication Prioritization Insights Clarivate 2025, Rationale current clinical indications and dosages of COVID 19 2025, Early phase clinical trial played a critical role in the Food and 2025, A standard database for drug repositioning Scientific Data 2025, Drug development Wikipedia 2025, Therapeutic Area vs. Indication Vial 2025, Drugs clinical trials of thyroid diseases in China 09 22 DDDT 2025, Drug misuse and dependence UK guidelines on clinical management 2025, What is a sham in clinical trials 2025, Classification Of Drugs and Clinical Therapeutic 2025, Drug Development and Safety Basicmedical Key 2025, PPT Teicoplanin and vancomycin clinical indications and 2025, Approved Drugs Archives Guideline Central 2025, Clinical Pharmacology powered by ClinicalKey Elsevier 2025, COVID Free Full Text A Comprehensive Review of Drug 2025, What is an IND What is a Clinical Hold Why Do Clinical Holds Happen 2025, Clinical trials in India Wikipedia 2025, US Drug Labels modules for clinical decision support 2025, Frontiers Trends in innovative pediatric drug development in 2025, Indication expansion Blockbuster Drugs Revolutionizing Treatment 2025, Prioritizing Indications An Analytical Framework Equinox Group 2025, PDF The New Antiepileptic Drugs Their Neuropharmacology and 2025, National Drug Authority Guidelines for the Conduct of Clinical Trials 2025, The Ethics of Off Label Drug Use Dimensions of Dental Hygiene 2025, Drug Development Boston Biotech Clinical Research 2025, Unintended Consequences of the Inflation Reduction Act Clinical 2025, Part1 chapter12 2025, PDR Guide to Drug Interactions Side Effects and Indications 2008 2025, Our Drug Pipeline Indication Strategy NW PharmaTech 2025, The Success Rate of Clinical Trials Is Higher than We Thought 2025, Special FDA designations for drug development orphan fast track 2025, Guideline for the management of pediatric off label use of drugs 2025, ReGARDD Regulatory Guidance for Academic Research of Drugs and 2025, Individual Approach to Laboratory Monitoring of Antiepileptic 2025.