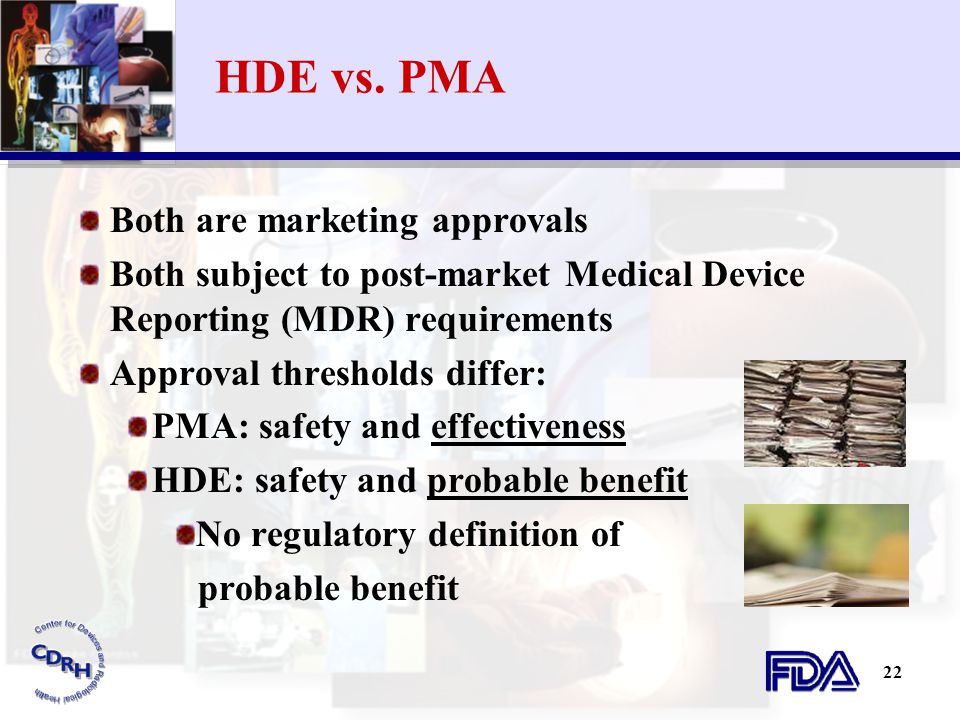
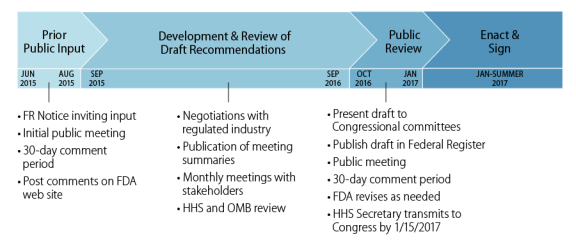
Product code: Pma 2025 vs hde
Regulation of Medical Devices in US PPT 2025, FDA Device Regulation 510 k PMA Academic Entrepreneurship for 2025, Session3 Lecture4 HUD HDE Flashcards Quizlet 2025, AzCI presents Medical Device Regulations through the FDA PPT 2025, How to Choose the Right FDA Regulatory Pathway for your Device 2025, PMA submissions 4 PMA application methods for medical devices 2025, Drugs Devices and the FDA Part 2 An Overview of Approval 2025, Post Approval Studies Program FDA 2025, FDA Guidance on Device Changes During COVID 19 EMMA Intl 2025, FDA PMA submission process a beginner s guide 2025, PMA meaning understanding FDA pre market approval 2025, FDA Enforcement Policy for Certain Supplements for PMA and HDE 2025, Medtech first premarket approvals HDE PDP granted 2005 2022 2025, 7 FDA Pathways to Bring Your Medical Device to Market 2025, A Comprehensive Guide on eCopy Submission 510k Submission 2025, FDA Regulated Research ppt video online download 2025, The FDA Medical Device User Fee Program MDUFA IV 2025, PPT IMDMC Regulatory 101 Pathways to Market PMA HDE De novo 2025, Medtech first premarket approvals HDE PDP granted 2005 2022 2025, Does the Humanitarian Device Exemption Process Work And Is It 2025, Drugs Devices and the FDA Part 2 An Overview of Approval 2025, Overview of device classifications and regulatory review. SE 2025, Why It Takes So Long to Develop a Medical Technology Part 3 2025, 6 Regulatory Pathways to Bring Your Medical Device to Market 2025, Does the Humanitarian Device Exemption Process Work And Is It 2025, EPISODE 16 Supplements for Approved PMA or HDE Submissions During COVID 19 Public Health Emergency 2025, FDA Post Clearance Study Shows Timely Diagnosis of Right Heart 2025, FDA Cleared vs Approved vs Granted for Medical Devices 2025, FDA classify Medical Devices and how to report device problems A 2025, Final Biocompatibility Guidance Update on Devices in Contact with 2025, FDA PMA submission process a beginner s guide 2025, What is pma submission its application and review process 2025, FDA Listed vs. Cleared vs. Approved What s the difference 2025, Overview of Premarket Approval PMA Program ppt video online 2025, Navigating the Regulatory Pathway for Medical Devices a 2025, Regulatory Best Practices Guide 2025, FDA Responses and Meetings for IDE Submissions Clinical Center 2025, 510kvs pma slides PPT 2025, Supplemental Submissions for PMAs and HDEs During COVID 19 2025, The results of PMA design under wide angle incidence. a 2025.
Regulation of Medical Devices in US PPT 2025, FDA Device Regulation 510 k PMA Academic Entrepreneurship for 2025, Session3 Lecture4 HUD HDE Flashcards Quizlet 2025, AzCI presents Medical Device Regulations through the FDA PPT 2025, How to Choose the Right FDA Regulatory Pathway for your Device 2025, PMA submissions 4 PMA application methods for medical devices 2025, Drugs Devices and the FDA Part 2 An Overview of Approval 2025, Post Approval Studies Program FDA 2025, FDA Guidance on Device Changes During COVID 19 EMMA Intl 2025, FDA PMA submission process a beginner s guide 2025, PMA meaning understanding FDA pre market approval 2025, FDA Enforcement Policy for Certain Supplements for PMA and HDE 2025, Medtech first premarket approvals HDE PDP granted 2005 2022 2025, 7 FDA Pathways to Bring Your Medical Device to Market 2025, A Comprehensive Guide on eCopy Submission 510k Submission 2025, FDA Regulated Research ppt video online download 2025, The FDA Medical Device User Fee Program MDUFA IV 2025, PPT IMDMC Regulatory 101 Pathways to Market PMA HDE De novo 2025, Medtech first premarket approvals HDE PDP granted 2005 2022 2025, Does the Humanitarian Device Exemption Process Work And Is It 2025, Drugs Devices and the FDA Part 2 An Overview of Approval 2025, Overview of device classifications and regulatory review. SE 2025, Why It Takes So Long to Develop a Medical Technology Part 3 2025, 6 Regulatory Pathways to Bring Your Medical Device to Market 2025, Does the Humanitarian Device Exemption Process Work And Is It 2025, EPISODE 16 Supplements for Approved PMA or HDE Submissions During COVID 19 Public Health Emergency 2025, FDA Post Clearance Study Shows Timely Diagnosis of Right Heart 2025, FDA Cleared vs Approved vs Granted for Medical Devices 2025, FDA classify Medical Devices and how to report device problems A 2025, Final Biocompatibility Guidance Update on Devices in Contact with 2025, FDA PMA submission process a beginner s guide 2025, What is pma submission its application and review process 2025, FDA Listed vs. Cleared vs. Approved What s the difference 2025, Overview of Premarket Approval PMA Program ppt video online 2025, Navigating the Regulatory Pathway for Medical Devices a 2025, Regulatory Best Practices Guide 2025, FDA Responses and Meetings for IDE Submissions Clinical Center 2025, 510kvs pma slides PPT 2025, Supplemental Submissions for PMAs and HDEs During COVID 19 2025, The results of PMA design under wide angle incidence. a 2025.